One not-to-discuss much discussion in the health and food relationship in the West is Alcohol Intolerance. Sometimes I comment on this at the Fundo Plate, especially in the videos.
Some people are curious and amazed when I comment that I have this too (yes, we are all wrong). So I think it’s a good opportunity to really explain what that is.
Let’s look at the Truth Pot: What is Alcohol Intolerance? In the end has the summary Very Long; I don’t read it, as always.
Is it an allergy?
Just like lactose intolerance: no, it is not an allergy. Again, these are situations produced by different systems of the body. In this case, it is involved with the metabolism (and elimination) of alcohol and not with the body’s immune system as are allergies. Can people be allergic to alcohol? You can. Chances are small, but it can. This is not the case here.
Combined?
What is Alcohol Intolerance?
We will start a correction: it is not called intolerance. The table is classified as an Dehydrogenase Aldehyde Deficiency (ALDH2). That is, there is something wrong in the operation of this such Adehyde Dehydrogenenasis 2.
Symptoms
The most visible of all: facial and body redness. In addition to these:
- Headache
- Weel and vomit
- Tachycardia
- It may worsen asthma picture
- Generalized malaise
To illustrate the scenario better: imagine the classic symptoms of hangover. But instead of appearing the next day, it happens immediately. I can assure you it’s not comfortable, it’s really bad.
It seemed more familiar, didn’t it? It is called in the Asian Glow gringa, but can also be called Asian Redness.
Then you will ask me: aldo-who?
Who is Dehydrogenase Aldehyde (ALDH2)?
In a very simple way: enzyme. So that tells us it’s a protein. It acts on the metabolization of alcohol (ethanol) when we drink either in the form of a drink and/or mixed in some dish.
This number “2” that accompanies the name indicates that there are other types of the enzyme with the same function. Generally, when we talk about enzymes, they are a group that have equal functions, but may have differences in structure, origin and where they are. In this case, ALDH2 is the main one.
For those interested, the gene responsible for the enzyme is located on chromosome 12 (12q24.12). The sequence has about 44 kbp (what pair bases) that processed results in a fragment with 517 amino acids. And after being produced, it lives in the mitochondrial matrix (read: within the mitochondria, an organelle that stays inside the cells).
To better understand her role, we need to know a little more about how the metabolization of alcohol happens.
The Metabolism of Alcohol
Virtually everything we consume needs to undergo some kind of transformation to be eliminated. With alcohol, it would be no different.
Alcohol is perceived as a toxic substance by the body, so it needs to be eliminated. To achieve this, alcohol is transformed and is responsible for the liver.
Although it is a simple organ in its constitution, it is of paramount importance: it is responsible for detoxification of the body. It is a continuous and constant work. And no, that weird green juice doesn’t have that function and it never will.
The basic process happens in two steps using two different main enzymes.
In the first, the enzyme Alcohol Deshydrogenase (ADH) acts first and turns alcohol into Acetaldehyde. So far, everything is fine. This is expected. But… it has an important detail.
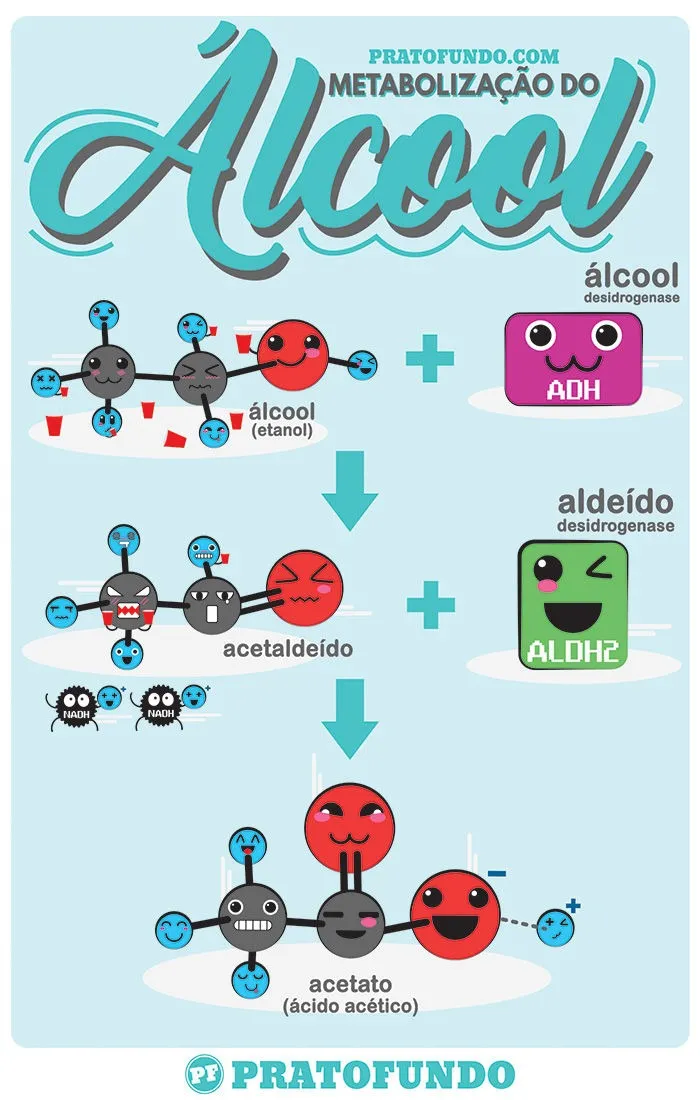
Acetaldehyde is more toxic than alcohol. It belongs to the group of aldehyde that has the organic function aldehyde: formed by a carbonyl center (carbon in double binding with oxygen) and a hydrogen. Did you take the name of the enzyme? A well-known substance of this group is the formaldehyde used in preservation of anatomical parts. But calm, not all aldehys are bad like this: the vanillin responsible for the aroma of vanilla also belongs to this group.
Therefore, acetaldehyde can’t stay in the body either, right?
The second step continues with: Acetaldehyde undergoes the action of the enzyme Aldehyde Dehydrogenase 2 (ALDH2) and becomes the Acetate radical (think of it as if it were acetic acid, the same as it has in vinegar) which is much less toxic in comparison and can be eliminated more easily with other processes of the body.
Are you still here with me? Great! Oh, great!
What I described is what I have been expected in most people. Oh, yes: the step by step is well simplified, the process uses other substances (such as cofactors) to be executed. Other metabolic pathways can happen, especially in the first stage, in cases of excess alcohol, there may be action of cytochrome P450 2E1 and catalase (another enzyme) to help with metabolism.
But of course there are the exceptions, the differences, right?
Deficiency of Aldehyde Dehydrogenase
I always say I would like to be a mutant. Actually, I am. Instead of gaining power, I lost.
Part of my ALDH2 is inactive, so my body can’t turn Acetaldehyde into the same rhythm as it’s produced (the other ALDH aren’t as effective as type 2). Thus, it has the accumulation of it in the body and as it is toxic, the symptoms felt are due to acetaldehyde poisoning. A mutation in the gene responsible for ALDH2 is the cause.
Just have a mutant gene to present this picture, as it has a semi-dominant characteristic to occur the reduction in activity. And when a person has both mutant genes (each on a chromosome 12), the enzyme has nothing activity.
The mutation is absurdly simple: there was a replacement of a measly amino acid at the 487 position of the enzyme, came out the glutamate and entered lysine. In essence it is that old story, just like when we take a recipe: it changes one ingredient and everything goes wrong. Here is the same thing.
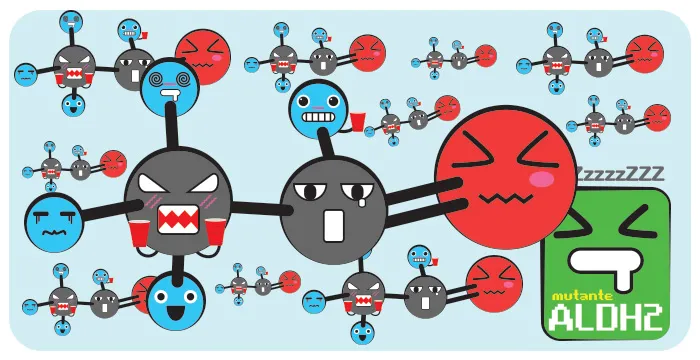
It is estimated that the condition is present in about 35-45% of the population of East Asia (Chinese, Japanese, Korean and Taiwanese).
Diagnosis
Generally, to determine this condition is done by an anamnesis of the patient. To confirm, there are blood tests to determine the content of acetaldehyde and, has patch alcohol test done on the skin that causes local erythema (redness).
And it’s not just that! What? Did you think it was over? No! No! Nothing is bad that can’t be made worse.
Alcohol Dehydrogenase
Do you know the first enzyme that starts the metabolism of alcohol, ADH? So there are variations of it that increase your activity. It can convert alcohol to higher speed acetaldehyde. Thus, the accumulation of Acetaldedehyde happens in a shorter time.
And in which population are these variations most found? He got it right who said: East Asians, Han Chinese, Japanese, Koreans, Filipinos and Malaysians.
Acetaldehyde
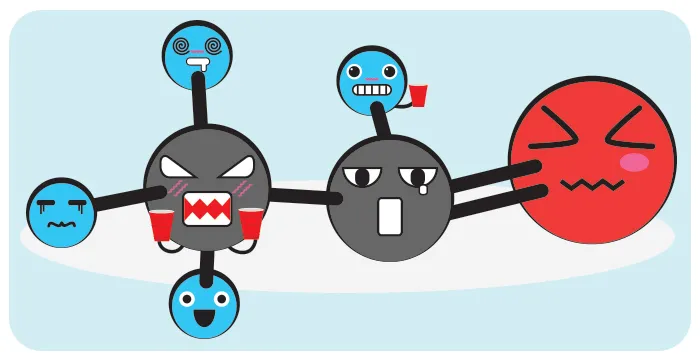
Remember I told you that it’s toxic? This result of the metabolization of alcohol is classified in Group 1 of Carcinogens by the International Agency for Research on Cancer, linked to the World Health Organization.
Group 1? What does that mean? You ask me, don’t you? This means: there is sufficient evidence that the substance is related to causing cancer. In this case, the main one is esophageal cancer, one of the deadliest with a survival rate ranging from 12% in Europe, 15% in the US and 31% in Japan.
A pause to give that dying internally.
What about me? What do I do?
So I think you could tell me that I have this condition, right? And I work with food, now what? It is almost the same situation as lactose intolerance: between getting sick and not tasting, what do we choose? Pass it wrong. I mean, more or less.
If I have the opportunity to taste some drink I have never had access to, it is quite possible that I will try it. But it is a sip or as it happens in wine tastings: I feel the taste, the notes and discards. I don’t really do I swallow. The discomfort caused is very unpleasant and has nothing to soften the situation.
In the oenology classes it was a certain suffering: good wines and some super expensive wines (which I will never buy) to try, and I with this detail. I only opened an exception in champagne and sparkling wine class, in which I tasted more than I should.
I, as a person, do not go to the market to buy alcoholic beverages to drink regularly for pleasure. It is the last thing that will provide me is pleasure, due to the discomfort generated. – I mean to? Honestly, no. However, I know that this part of alcoholic beverages in my training as a cook is not the best because I cannot build a taste-of-slweal memory that is only possible by experimenting.
Very Long; Not Li
- It is not an allergy. Are we combined?
- It is a deficiency in an enzyme called Aldehyde Dehydrogenase 2.
- The enzyme mutated and became inactive.
- About 35-50% of the East Asian population has this mutation.
- It is necessary in the metabolization of alcohol, but as it does not work a toxic substance called Acetaldehyde stays longer in the body.
- What causes the symptoms: facial and body redness, headache, nausea, vomiting, tachycardia and general malaise.
- It is a hangover that happens at the same time as alcohol consumption.
- This acetaldehyde produced in the metabolism of alcohol is associated with cases of cancer, mainly of the esophagus.
- It has no cure, some experiments have been done, but nothing is available to change the condition.

Bibliography
- BROOKS, P. J. et al. The Alcohol Flushing Response: An Unrecognized Risk Factor for Esophageal Cancer from Alcohol Consumption. PLoS Medicine, v. 6, n. 3, sea. 2009. 2009.
- CHEN, C.-H. et al. Targeting Aldehyde Dehydrogenase 2: New Therapeutic Opportunities. Physiological Reviews, see. 94, n. 1, p. 1–34, Jan. 2014. 2014. I’ve a’d it a year a year shot and a long shot a’d of a’s a’s a’s a’s a year the one.
- ENG, M. Y.; LUCZAK, S. E.; WALL, T. L. ALDH2, ADH1B, and ADH1C genotypes in Asians: a literature review. Alcohol Research & Health: The Journal of the National Institute on Alcohol Abuse and Alcoholism, v. 30, n. 1, p. 22–27, 2007.
- ENOMOTO, N.; TAKADA, A.; DATE, T. Genotyping of the aldehyde dehydrogenase 2 (ALDH2) gene using the polymerase chain reaction: evidence for single point mutation in the ALDH2 gene of ALDH2-deficiency. Gastroenterology Japonica, v. 26, n. 4, p. 440-447, Aug. 1991..
- IARC. International Agency for Research on Cancer. Agents classified by the IARC monographs, v. 1–117, 2016.
- Lehninger, Albert L.; Nelson, David L.; COX, Michael M. Lehninger Principles of Biochemistry. New York: W.H. Freeman and Company, 2007. (English)
- MATSUO, K. et al. The aldehyde dehydrogenase 2 (ALDH2) Glu504Lys polymorphism interacts with alcohol drinking in the risk of cancer. Carcinogenesis, v. 34, n. 7, p. 1510–1515, Jul. 2013.
- McGEE, Harold. On food and cooking: the science an lore of the kitchen. New York: Scribner, 2004. (English | English)
- NCBI: Gene. ALDH2 aldehyde dehydrogenase 2 family (mitochondrial) (Homo sapiens (human) – Gene – NCBI. Available at: https://www.ncbi.nlm.nih.gov/gene/217. Accessed on: 23 Jan. 2017. 2017.
- Overview: How Is Alcohol Metabolized by the Body? NIAAA Publications. Available at: htps://pubs.niaaa.nih.gov/publications/arh294/245-255.htm. Access on: 24 Jan. 2017. 2017.
- PENG, G.-S.; YIN, S.-J. Effect of the allelic variants of aldehyde dehydrogenase ALDH2 2 and alcohol dehydrogenase ADH1B2 on blood acetal de concentrationshyde. Human Genomics, v. 3, n. 2, p. 121–127, 1 Jan. 2009. 2009.
- TOMITA, Y. et al. Effects of chronic ethanol intoxication on aldehyde dehydrogenase in mouse liver. Alcohol and Alcoholism (Oxford, Oxfordshire), v. 27, n. 2, p. 171–180, sea. 1992.
- WANG, X. et al. Heterotetramers of human liverchondrial (class 2) alde dehydrogenase expressed in Escherichia coli a model to study the heterotetramers expected to be found in oriental people. Journal of Biological Chemistry, v. 271, n. 49, p. 31172–31178, 6 Dec. 1996.

Sign up for our newsletter and stay up to date with exclusive news
that can transform your routine!
Warning: Undefined array key "title" in /home/storelat/public_html/wp-content/plugins/link-whisper-premium/templates/frontend/related-posts.php on line 12
Warning: Undefined array key "title_tag" in /home/storelat/public_html/wp-content/plugins/link-whisper-premium/templates/frontend/related-posts.php on line 13