Realize how thermodynamics is part of our lives, based on understanding the reactions between heat, work and energy.
Thermodynamics is an area of physics that focuses on studying energy transfers through the relationships between heat, energy and work. This observation occurs through the analysis of phenomena in which there are exchanges in the amount of heat, as well as an exchange in the work used in a physical process.
This area of physics, characterized as thermodynamics, was developed by researchers, still during the industrial revolution, who were looking for ways to mechanically improve machines. In short, given the situation at the time, good machine performance was essential for consolidating the great change that mechanization means to this day.
However, when we take into account all the devices present in our daily lives, we realize how this phenomenon is more present than ever, for example, in thermal machines and refrigerators. Above all, this phenomenon is present in the kitchen.
Finally, the fundamental laws of thermodynamics present the relationship between how heat becomes work and vice versa. That is, how energy is generated or used in a given system.

Fundamental concepts
- Heat: is the exchange of thermal energy between bodies, that is, it is the energy used that one body deposits in another;
- Energy: refers to the amount of work used by a body to carry out a movement;
- Temperature: concerns the agitation of the molecules. As a result, the hotter the more agitated; the less heat, the less agitation;
- Volume: refers to the space occupied by the molecules under analysis;
- Pressure: Pressure is the result of the movement of particles in a container.
1st law of thermodynamics
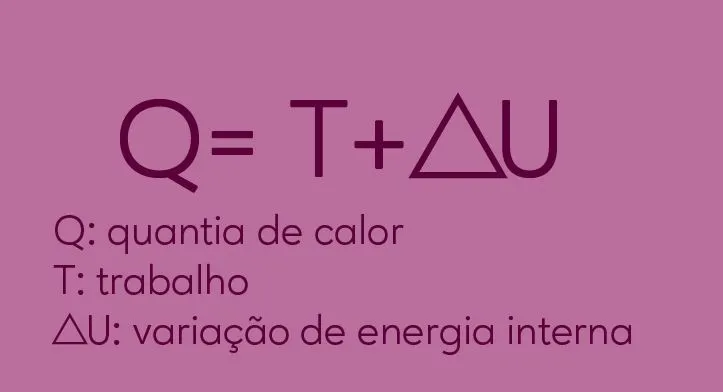
Firstly, this first law is based on the principle of conservation of energy. In other words, the energy that is present in a system cannot be destroyed or created. This energy is only transformed through the work used in this system and the amount of heat. Your equation is:
2nd law of thermodynamics
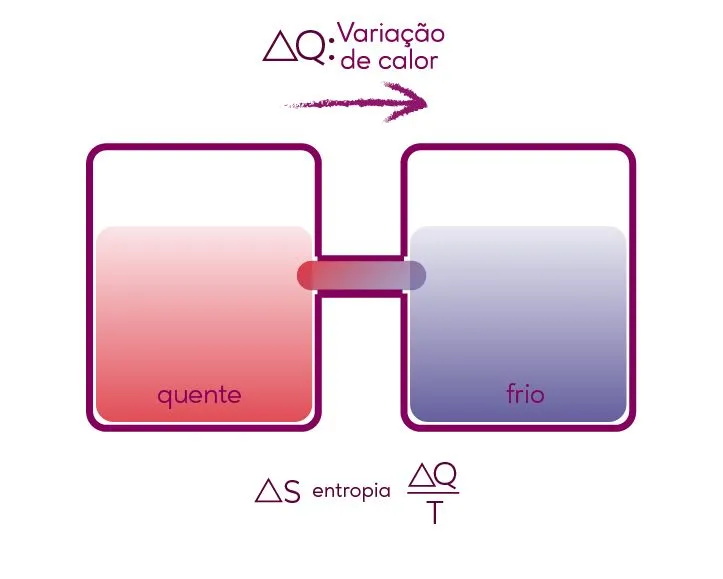
This law is based on the observation of spontaneous heat transfer. Also known as entropy, this heat transfer always occurs from the hotter body to the colder body, and this process is irreversible. Heat cannot be fully converted into another, because it is a degraded form of energy. When used, it dissipates.
Lei zero
This law is called the zeroth law because it is fundamental to the other two previous laws. This law is the basis for the condition to obtain a form of thermal equilibrium. According to the 1st law, the heat that is used can only transform, that is, degrade; since, as stated in the 2nd law, heat transfer is irreversible.
Two bodies at different temperatures, when in contact, the temperature of the hotter one predominates, which dissipates to the colder one until the temperatures equalize, reaching thermal equilibrium as a result.
3rd law of thermodynamics
This law is a source of controversy for some researchers, since, despite being called a law, it is more similar to a rule. The 3rd law of thermodynamics is a way of trying to establish an absolute point of reference as a benchmark for entropy; that is, a reference point for the degree of disorder of a system, calculated by the 2nd law of thermodynamics.
From a lower temperature limit (absolute zero), this law says that it is not possible for a substance to reach the temperature of absolute zero. It is from this perspective that it is possible to verify implications regarding the performance of thermal machines that have never reached a position of efficiency equal to 100%.
thermodynamic system
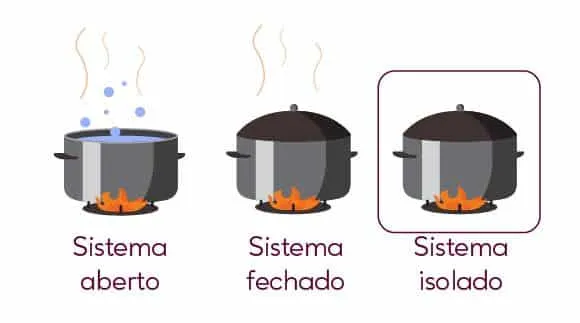
The system, in thermodynamics, concerns the space in which one or more bodies relate to the ‘environment’ or ‘universe’ external to that system, which can be closed, open or isolated. If open, there is an exchange of heat and mass with the environment; Once closed, there is only heat exchange. And when isolated, there is no exchange of heat or mass.
Thermal state
The thermal state concerns the behavior of molecules; these are evaluated by gas molecules called ideal gases. The thermal state is more easily evaluated in gases than in other physical states, such as liquids and solids.
Ideal gases are scenarios in which molecules move chaotically and only interact in their collisions, with these collisions being considered ‘elastic’, as they last a short time. This assessment already brings other quantities into play: pressure, volume and temperature.
Thermodynamics in everyday life
Now that you know how the theory of thermodynamics works, how about (re)acquainting yourself with some everyday habits that are the pure practice of all these theories?!
The dreaded chocolate tempering
Also known as Bain Maria, this gradual change of temperatures is the practice of the 2nd of thermodynamics.

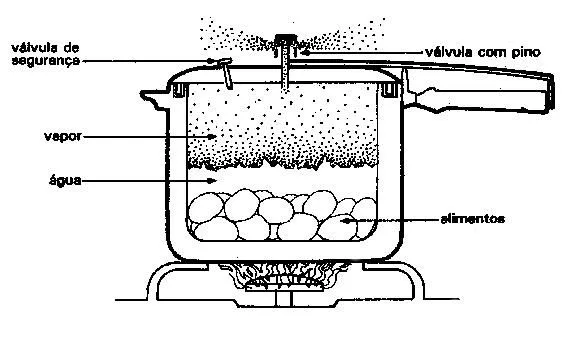
The pressure cooker
The pressure cooker reduces the temperature and mass output, causing the pressure to increase with the agitation of the molecules, arising from the temperature used with the stove fire.
A thermos carafe
Its original name is ‘Dewar vase’, but popularly it became known as Thermal Bottle. It consists of a container that achieves practically perfect thermal insulation. As a result, it is able to have a prolonged thermal preservation capacity because it avoids the exchange of heat with the external environment.

Finally, did you like this article? Then you will also like this: Pressure cooker – How it works, how to use, care and recipes
Images: Ajinomoto Flavors. Unsplash, help in the kitchen.
Sources: Descomplica, Toda materia, Brasil Escola, Prepara Enem.

Sign up for our newsletter and stay up to date with exclusive news
that can transform your routine!
Warning: Undefined array key "title" in /home/storelat/public_html/wp-content/plugins/link-whisper-premium/templates/frontend/related-posts.php on line 12
Warning: Undefined array key "title_tag" in /home/storelat/public_html/wp-content/plugins/link-whisper-premium/templates/frontend/related-posts.php on line 13




